Amino acids are the building blocks of proteins and play a crucial role in various biological processes. They are vital for the structure, function, and regulation of tissues and organs.
Each amino acid has an amino group, a carboxyl group, and a hydrogen atom. It also features a unique side chain (R group) attached to a central carbon atom.
Definition and Importance
Amino acids serve as precursors to proteins and other biomolecules like hormones and neurotransmitters. They are indispensable for growth, repair, and maintaining the body’s metabolic balance.
Role in Biochemistry
In biochemistry, amino acids are fundamental to enzymatic reactions, gene expression, and cellular communication. They influence nearly all physiological processes.
General Structure
Each amino acid shares a common backbone structure but differs in its R group. This difference determines the amino acid’s chemical properties.
Classification of Amino Acids
1. Based on Side Chain Properties
Nonpolar Amino Acids
Nonpolar amino acids have hydrophobic side chains and are typically found in the interior of proteins. Examples: Glycine, Alanine, Valine.
Polar Uncharged Amino Acids
These amino acids have hydrophilic side chains that can form hydrogen bonds. Examples: Serine, Threonine.
Positively Charged (Basic) Amino Acids
Basic amino acids have side chains that carry a positive charge at physiological pH. Examples: Lysine, Arginine.
Negatively Charged (Acidic) Amino Acids
These have side chains that are negatively charged at physiological pH. Examples: Aspartic acid, Glutamic acid.
Aromatic Amino Acids
Aromatic amino acids contain a benzene ring in their side chain. Examples: Phenylalanine, Tyrosine.
2. Based on Nutritional Need
Essential Amino Acids
Can’t be synthesized by the body and must be obtained from diet. Examples: Leucine, Methionine.
Non-Essential Amino Acids
Synthesized by the body in sufficient quantities. Examples: Alanine, Glutamate.
Conditionally Essential Amino Acids
Become essential under specific physiological conditions. Examples: Arginine, Glutamine.
3. Based on Metabolism
Glucogenic Amino Acids
Can be converted into glucose through gluconeogenesis. Examples: Alanine, Serine.
Ketogenic Amino Acids
Degraded into ketone bodies. Examples: Leucine, Lysine.
Both Glucogenic and Ketogenic
Examples: Isoleucine, Phenylalanine.
Structure and Properties of Amino Acids
1. General Structure
Amino Group
Participates in peptide bond formation.
Carboxyl Group
Provides the acidic property of amino acids.
R-Group (Side Chain)
Determines the unique characteristics of each amino acid.
Alpha Carbon
The central carbon to which all groups are attached.
Chirality and Stereochemistry
Most amino acids exhibit chirality, existing in L- and D-forms.
2. Physical Properties
Solubility
Depends on the polarity of the side chain.
Isoelectric Point (pI)
The pH at which the amino acid carries no net charge.
Optical Activity
Chiral amino acids rotate plane-polarized light.
3. Chemical Properties
Acid-Base Behavior
Amino acids act as buffers in physiological systems.
Reactivity of Functional Groups
Key to peptide bond formation and metabolic reactions.
Formation of Peptide Bonds
Amino acids link through peptide bonds to form proteins.
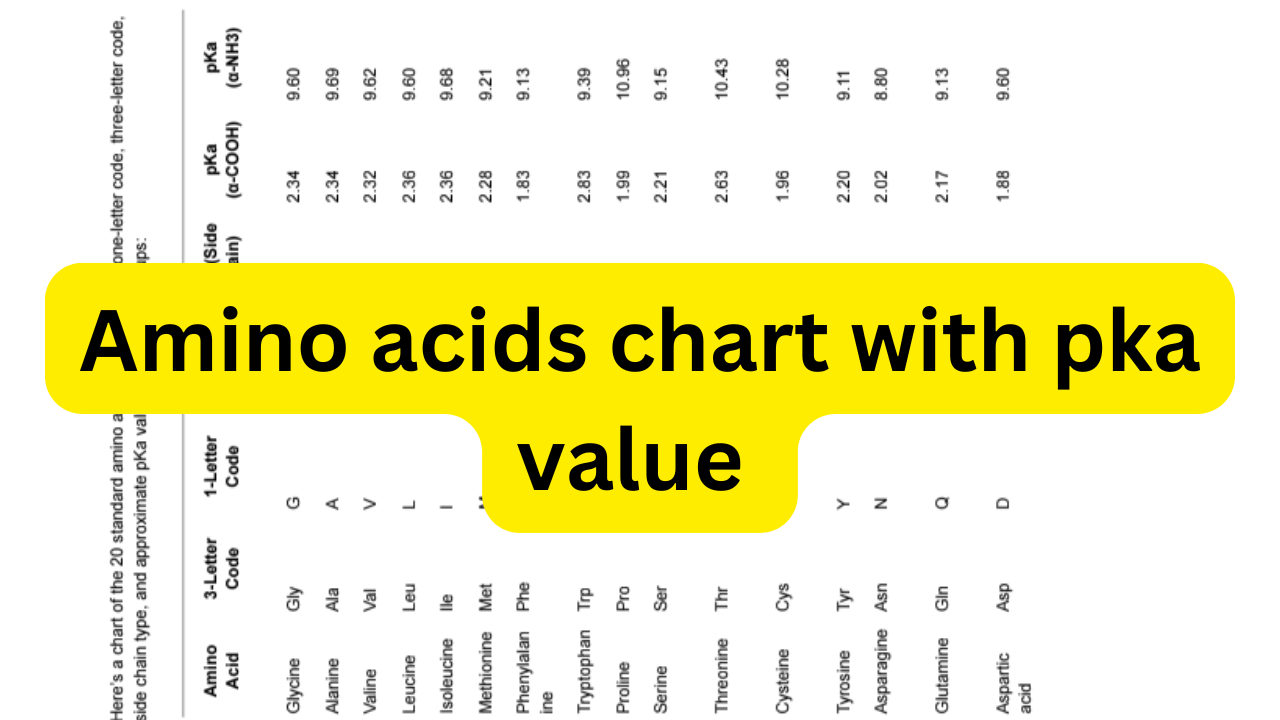
Importance of Pka value in Amino acid chart
The pKa value in the amino acid chart indicates the acid dissociation constant. It applies to specific functional groups within the amino acid molecule. It indicates the pH at which a given group (carboxyl group, amino group, or ionizable side chain) is 50% ionized.
Amino acids chart with pka pdf file
Amino acids chart with pka
Understanding these values is critical in biochemistry. They decide how amino acids behave under different pH conditions. This is particularly important in protein folding and enzyme activity.
Key pKa Values in Amino Acids:
- pKa of the Carboxyl Group (α-COOH):
- Found in all amino acids.
- Generally has a pKa around 2.0–2.5, meaning the carboxyl group is negatively charged above this pH.
- pKa of the Amino Group (α-NH3):
- Found in all amino acids.
- Typically has a pKa around 9.0–10.5, meaning the amino group is positively charged below this pH.
- pKa of Ionizable Side Chains (if present):
- Applies to amino acids with ionizable R-groups (side chains).
- Examples include:
- Aspartic acid (pKa ~ 3.65): Side chain is negatively charged above this pH.
- Glutamic acid (pKa ~ 4.25): Side chain is negatively charged above this pH.
- Histidine (pKa ~ 6.00): Side chain can act as a buffer near physiological pH.
- Cysteine (pKa ~ 8.18): Important in forming disulfide bonds in proteins.
- Tyrosine (pKa ~ 10.07): Can be deprotonated at high pH.
- Lysine (pKa ~ 10.53): Side chain remains positively charged in physiological conditions.
- Arginine (pKa ~ 12.48): Strongly basic and almost always positively charged in biological systems.
Why pKa Values Matter:
- Protein Structure: pKa values influence how amino acids interact and fold in proteins.
- Buffering Capacity: Certain amino acids, like histidine, act as natural pH buffers due to their pKa values near physiological pH (~7.4).
- Enzyme Function: Enzymes often depend on the ionization state of amino acid side chains in their active sites for catalysis.
Functions of Amino Acids
1. Protein Synthesis
Amino acids are the monomers for proteins, which perform structural, enzymatic, and signaling functions.
2. Precursors for Biomolecules
Neurotransmitters
Amino acids like tryptophan and tyrosine are precursors for serotonin and dopamine.
Hormones
For example, tyrosine is the precursor for thyroid hormones.
Nucleotides
Amino acids contribute to the synthesis of DNA and RNA.
3. Energy Metabolism
Catabolism
Amino acids are broken down for energy, especially during fasting.
Role in the Krebs Cycle
Many amino acids feed into the Krebs cycle, supporting ATP production.
4. Specialized Roles
Detoxification (Glutathione)
Amino acids like cysteine play a role in detoxifying harmful substances.
Osmoregulation
Amino acids help maintain cellular osmotic balance.
Conclusion
Recap of Key Points
Amino acids are central to life, serving as the building blocks of proteins and playing versatile roles in metabolism, signaling, and repair.
Future Research Directions
Advancements in amino acid-related therapies and biotechnology hold great promise.
Importance of Amino Acids in Life Sciences
Amino acids’ multifaceted roles make them indispensable in understanding biological systems and developing medical innovations.