Osmosis and diffusion are two fundamental processes by which molecules move and substances are transported. While they may seem similar at first glance, they are distinct in their mechanisms, purposes, and the types of molecules they involve.
Here is the key differences between osmosis and diffusion, shedding light on their importance in both natural and artificial systems.
What is Diffusion?
Diffusion is the movement of molecules from an area of higher concentration to an area of lower concentration. This process continues until the concentration of the molecules is equal throughout the available space, achieving equilibrium. It can occur in gases, liquids, or solids and does not require a semi-permeable membrane.
- Example: The fragrance of perfume spreading through a room, or oxygen moving from the air in the lungs into the bloodstream.
Characteristics of Diffusion
- It can involve gases, liquids, or dissolved solutes.
- Molecules mrom higher to lower concentration.
- It is passive process, meaning no external energy (ATP) is required.
- It is generally faster in gases than in liquids and solids due to differences in particle movement.
What is Osmosis
Osmosis is a specific type of diffusion that involves the movement of water molecules across a semi-permeable membrane. Water moves from an area of lower solute concentration to an area of higher solute concentration. It is a crucial in maintaining the balance of fluids in living organisms.
- Example: Water entering plant roots from the soil, or water moving in and out of cells to maintain osmotic pressure.
Characteristics of Osmosis
- Type of Molecule: Involves only water molecules.
- Direction of Movement: From an area of higher water concentration (lower solute concentration) to lower water concentration (higher solute concentration).
- Energy Requirement: Passive process, requiring no external energy.
- Membrane Requirement: Requires a semi-permeable membrane that allows only water to pass through while preventing solutes from moving.
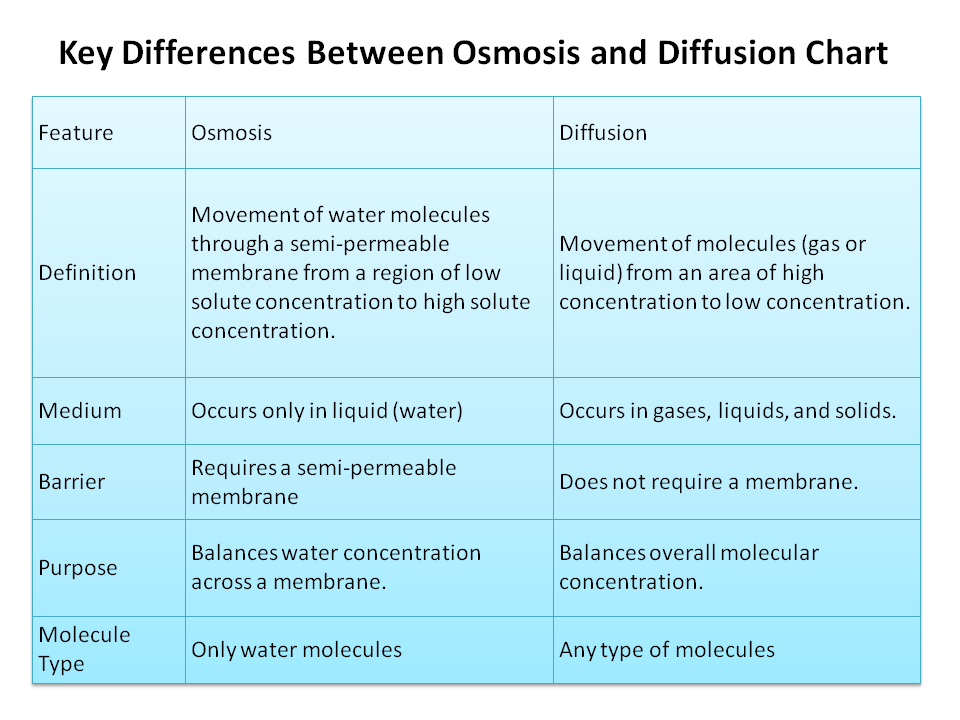
Key Differences Between Osmosis and Diffusion Chart
| Definition | Movement of molecules from high to low concentration. | Movement of water across a semi-permeable membrane from low to high solute concentration. |
| Molecule Involved | Gases, liquids, or solutes. | Only water molecules. |
| Membrane Requirement | Can occur with or without a membrane. | Requires a semi-permeable membrane. |
| Direction of Movement | Down the concentration gradient. | From high water concentration to low water concentration. |
| Energy Requirement | Passive (no energy required). | Passive (no energy required). |
| Example | Oxygen diffusing into cells. | Water entering plant roots from soil. |
What are the Biological Importance of diffusion and osmosis?
Both osmosis and diffusion play critical roles in biological systems:
- Diffusion is essential for processes like gas exchange in the lungs, nutrient absorption in the intestines, and the transport of small molecules within cells.
- Osmosis is vital for maintaining cell turgor in plants, regulating fluid balance in animal cells, and controlling blood pressure by balancing water levels in tissues.