Hemoglobin is a vital protein in red blood cells responsible for transporting oxygen from the lungs to tissues and carrying carbon dioxide back to the lungs for exhalation.
While most people are familiar with adult hemoglobin (HbA), the human body also produces a distinct form called fetal hemoglobin (HbF) during fetal development.
Here’s the key differences between fetal and adult hemoglobin, highlighting their structures, functions, and clinical significance.
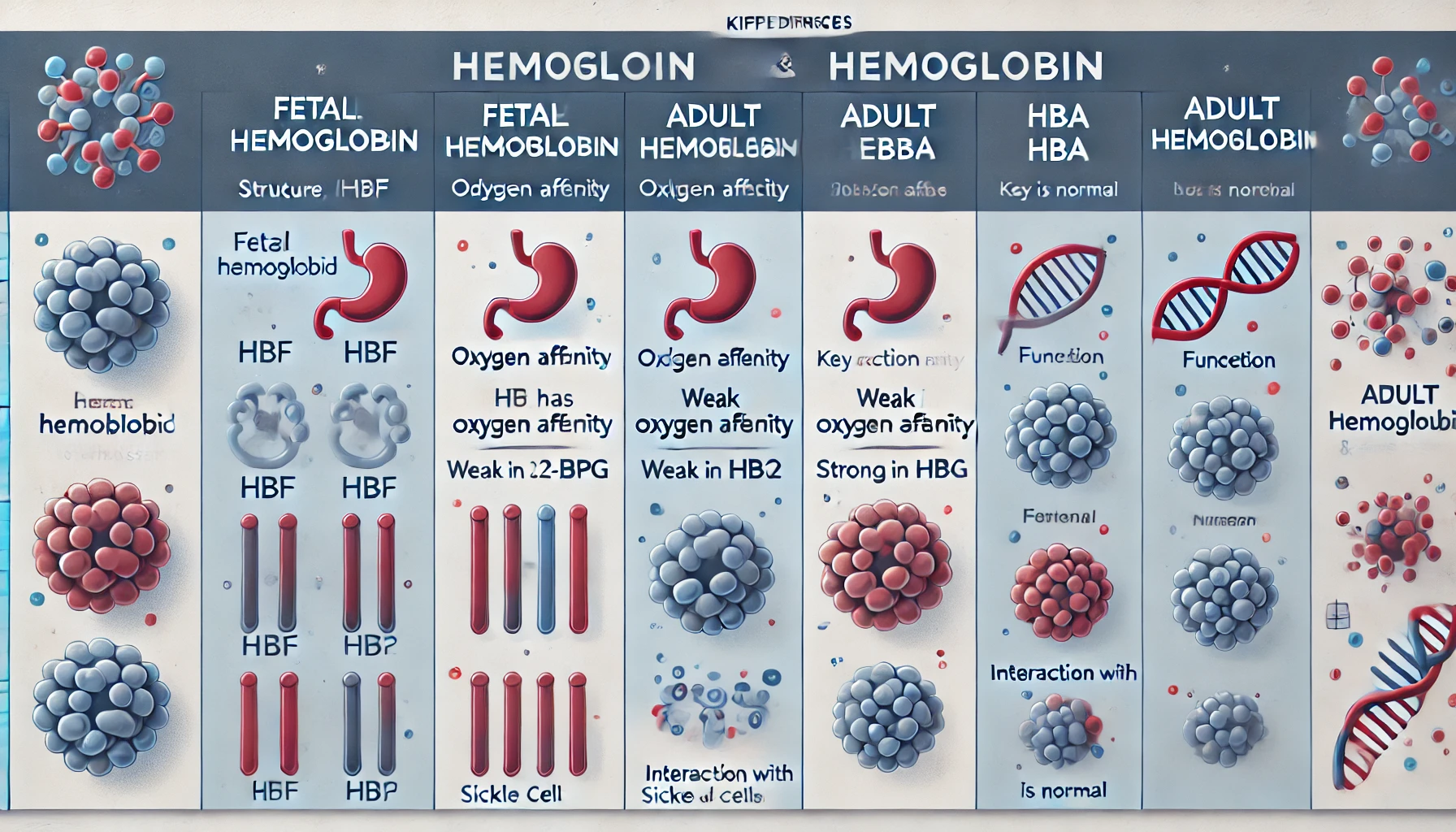
1. Structural Differences
What is Fetal Hemoglobin (HbF)?
- Fetal hemoglobin consists of two alpha (α) chains and two gamma (γ) chains, making its molecular structure α2γ2.
- The gamma chains in HbF are encoded by genes on chromosome 11 and are primarily expressed during fetal development.
What is Adult Hemoglobin (HbA)?
- Adult hemoglobin is composed of two alpha (α) chains and two beta (β) chains, forming the structure α2β2.
- The beta chains are also encoded by genes on chromosome 11 but are expressed after birth, as gamma chain production decreases.
2. Oxygen Affinity differences
One of the most critical differences between fetal and adult hemoglobin is their affinity for oxygen.
- Fetal hemoglobin has a significantly higher affinity for oxygen compared to adult hemoglobin. This is essential for fetal development because the fetus must extract oxygen from the mother’s blood across the placenta.
- Fetal hemoglobin is less sensitive to changes in pH and carbon dioxide levels, allowing it to maintain a high oxygen affinity even in the relatively low-oxygen environment of the placenta.
Mechanism of High Oxygen Affinity
The higher oxygen affinity of HbF is due to its reduced interaction with 2,3-bisphosphoglycerate (2,3-BPG), a molecule that normally binds to adult hemoglobin and reduces its affinity for oxygen. This weak interaction allows HbF to retain oxygen more effectively.
3. Functional Role differences
Fetal Hemoglobin (HbF)
- HbF functions primarily during fetal life, ensuring efficient oxygen transfer from the mother to the fetus.
- HbF begins to be produced around the 8th week of gestation and remains the dominant form of hemoglobin until shortly after birth.
- After birth, the production of gamma chains decreases, and beta chain synthesis increases, leading to a gradual replacement of HbF with HbA.
Adult Hemoglobin (HbA)
- HbA is the predominant hemoglobin in healthy children and adults, optimized for oxygen delivery in tissues under normal physiological conditions.
- HbA is better suited for oxygen release in tissues, where oxygen demand is higher and the environment is less oxygen-rich.
4. Clinical Significance differences
Persistence of Fetal Hemoglobin
In some individuals, fetal hemoglobin production persists into adulthood, a condition known as hereditary persistence of fetal hemoglobin (HPFH). While typically asymptomatic, this condition can influence the severity of hemoglobinopathies like sickle cell disease and beta-thalassemia.
Therapeutic Applications
Fetal hemoglobin has garnered attention for its potential therapeutic benefits:
- Increased HbF levels can mitigate the severity of these disorders by reducing the concentration of defective adult hemoglobin.
- This medication stimulates HbF production and is used to treat sickle cell disease by decreasing the frequency of vaso-occlusive crises and improving overall oxygen transport.
5. Key Differences between fetal hemoglobin and Fetal Hemoglobin (HbF) table
| Feature | Fetal Hemoglobin (HbF) | Adult Hemoglobin (HbA) |
|---|---|---|
| Structure | α2γ2 | α2β2 |
| Oxygen Affinity | Higher | Lower |
| 2,3-BPG Interaction | Weak | Strong |
| Developmental Role | Fetal oxygen transport | Postnatal oxygen delivery |
| Clinical Relevance | Protective in hemoglobinopathies | Normal oxygen transport |