The Osazone test is typically used to identify reducing sugars based on the formation of crystalline derivatives known as osazones.
However, sucrose is a non-reducing sugar (it does not have a free aldehyde or ketone group in its structure under normal conditions), and therefore, it does not form osazones directly when subjected to this test.
Principle of Osazone Test
The osazone test is based on the reaction of reducing sugars with phenylhydrazine under heated conditions. Reducing sugars possess a free aldehyde (-CHO) or keto (-C=O) group capable of reducing metal ions or reacting with phenylhydrazine.
Osazone Test Reaction Mechanism:
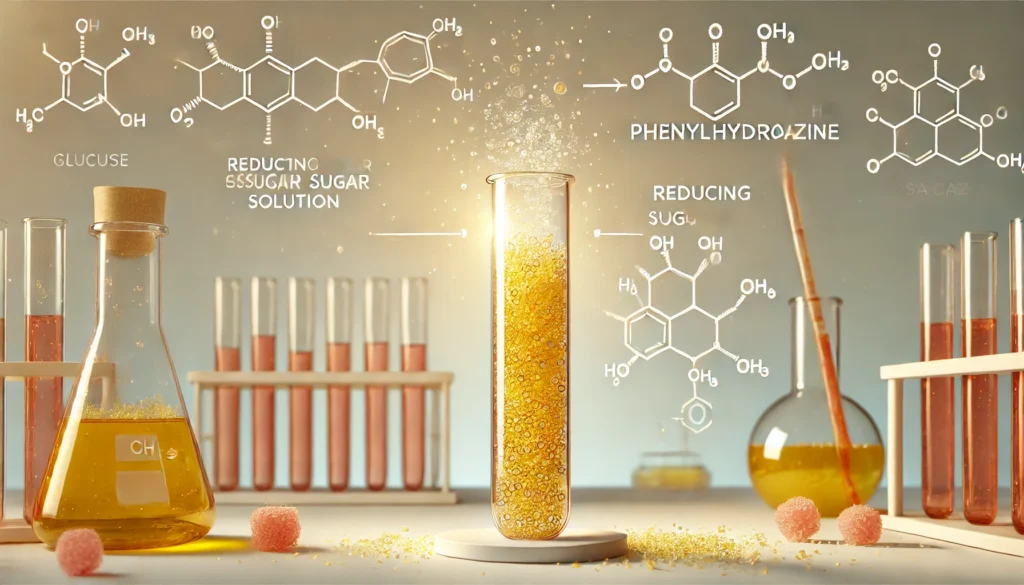
- Formation of Phenylhydrazone: The carbonyl group (C=O) of the reducing sugar reacts with phenylhydrazine, forming a hydrazine derivative.
- Formation of Osazone : In the presence of excess phenylhydrazine and heat, the hydrazone undergoes further reaction at the carbon atoms adjacent to the carbonyl group, forming a yellow-colored osazone crystal.
NOTE: This reaction is specific for sugars with free carbonyl groups at the C1 and C2 positions.
Osazone Test procedure
Materials Required
- Sugar solution (e.g., glucose, fructose, lactose)
- Phenylhydrazine hydrochloride
- Sodium acetate
- Glacial acetic acid
- Water bath
- Test tubes
- Microscope (for observing crystals)
Procedure Steps
- Mix equal parts of phenylhydrazine hydrochloride, sodium acetate, and glacial acetic acid to prepare the osazone reagent.
- Take 5 mL of the sugar solution (5% concentration) in a clean test tube.
- Add 1 mL of the prepared osazone reagent to the sugar solution.
- Place the test tube in a boiling water bath and heat it for about 10-15 minutes.
- Allow the test tube to cool slowly to room temperature.
- Examine the precipitate under a microscope to observe the characteristic osazone crystals.
Result Interpretation
Different sugars produce osazone crystals with unique shapes, which help identify the specific sugar present in the sample.
| Sugar | Osazone Crystal Shape | Time of Formation |
|---|---|---|
| Glucose | Needle-shaped crystals | 5-10 minutes |
| Fructose | Needle-shaped crystals (similar to glucose) | 5-10 minutes |
| Maltose | Sunflower-shaped crystals | 10-15 minutes |
| Lactose | Powder puff or Hedgehog-shaped crystals | 15-20 minutes |
| Galactose | Needle-shaped crystals | 5-10 minutes |
Key Points
- Glucose and Fructose form identical needle-shaped crystals because both are aldoses (at C1 and C2) or ketoses.
- Maltose forms sunflower-shaped crystals due to its disaccharide structure.
- Lactose produces hedgehog-shaped crystals, helping differentiate it from other sugars.
Applications of Osazone Test
- Identification of Carbohydrates: Differentiates between glucose, fructose, lactose, and maltose.
- Medical Diagnostics: Used historically in the diagnosis of diseases like diabetes mellitus by identifying the presence of reducing sugars in urine.
- Biochemistry Education: A teaching tool in laboratories to demonstrate carbohydrate chemistry.
Limitations of Osazone Test
- The test is not specific for a single sugar, as some sugars form similar crystal shapes (e.g., glucose and fructose).
- Non-reducing sugars (e.g., sucrose) do not react in this test.
- Requires careful observation under a microscope to correctly identify crystal morphology.
Conclusion
The Osazone test is a simple yet effective biochemical test for the differentiation of reducing sugars. Despite being replaced by modern analytical techniques like chromatography and spectrophotometry, it remains a valuable educational tool in carbohydrate chemistry.

1 thought on “Osazone test for sucrose principle procedure and result”