Column chromatography is a widely used separation technique in chemistry. It involves packing a vertical glass or plastic column with a solid stationary phase, often silica gel or alumina.
The compounds in the mixture move at different rates through the column based on their interactions with the stationary phase.
How does column chromatography work
Column chromatography works through a series of following steps:
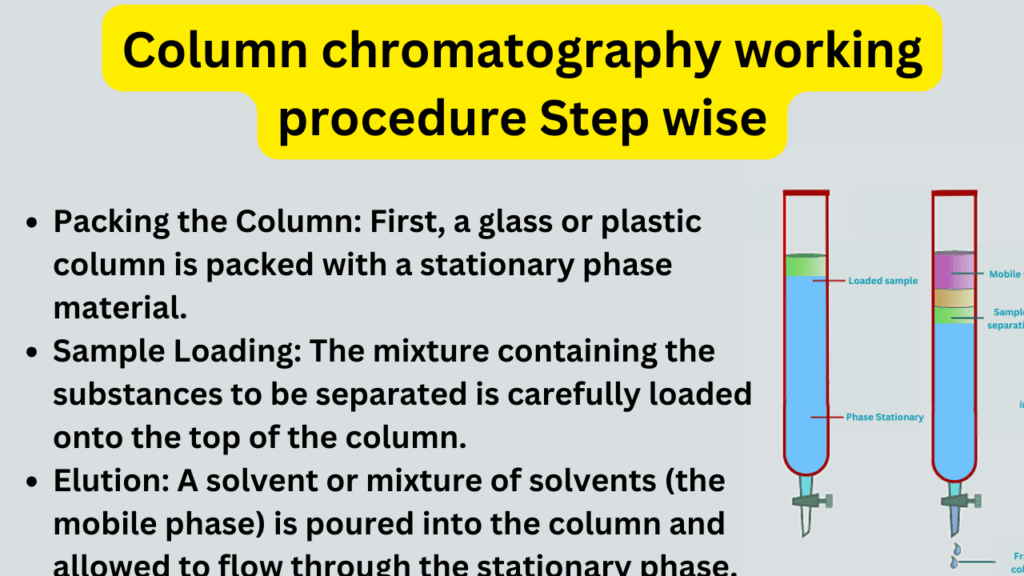
- Packing the Column: First, a glass or plastic column is packed with a stationary phase material, such as silica gel or alumina. This creates a solid bed within the column.
- Sample Loading: The mixture containing the substances to be separated is carefully loaded onto the top of the column. This is often done using a pipette, and the sample is typically dissolved in a suitable solvent.
- Elution: A solvent or mixture of solvents (the mobile phase) is poured into the column and allowed to flow through the stationary phase. The mobile phase moves downward due to gravity.
- Separation: As the mobile phase travels through the column, the different compounds in the sample interact differently with the stationary phase. Compounds with stronger affinity for the stationary phase move more slowly, while those with weaker interactions move faster.
- Collection of Fractions: As the mobile phase drips out of the column’s bottom, it carries the separated compounds with it. These compounds are collected in fractions using test tubes or vials. Each fraction may contain one or more compounds.
- Analysis: The collected fractions are then analyzed using various techniques, such as TLC (Thin-Layer Chromatography) or spectroscopy, to identify and quantify the separated compounds.
- Recovery: If needed, the desired compound(s) can be further purified by repeating the chromatography process or concentrating the fractions containing the target compound.