Normal saline is commonly used in medical settings for various purposes, including hydration and medication delivery. Sometimes, a diluted solution like 0.45% saline is needed for specific treatments.
This guide will walk you through the process of preparing 0.45% normal saline from a 0.9% saline solution.
Materials required
- 0.9% Normal Saline: This is your starting solution.
- Sterile Water: Used for dilution.
- Measuring Equipment: Graduated cylinder or syringe for precise measurement.
- Mixing Container: A sterile bottle or beaker.
- Labels: For proper identification of your solution.
NOTE:
- 0.9% Saline: This solution contains 9 grams of sodium chloride (NaCl) in 1 liter of water.
- 0.45% Saline: This solution contains 4.5 grams of sodium chloride in 1 liter of water.
To prepare 0.45% saline, you will dilute the 0.9% saline solution with sterile water.
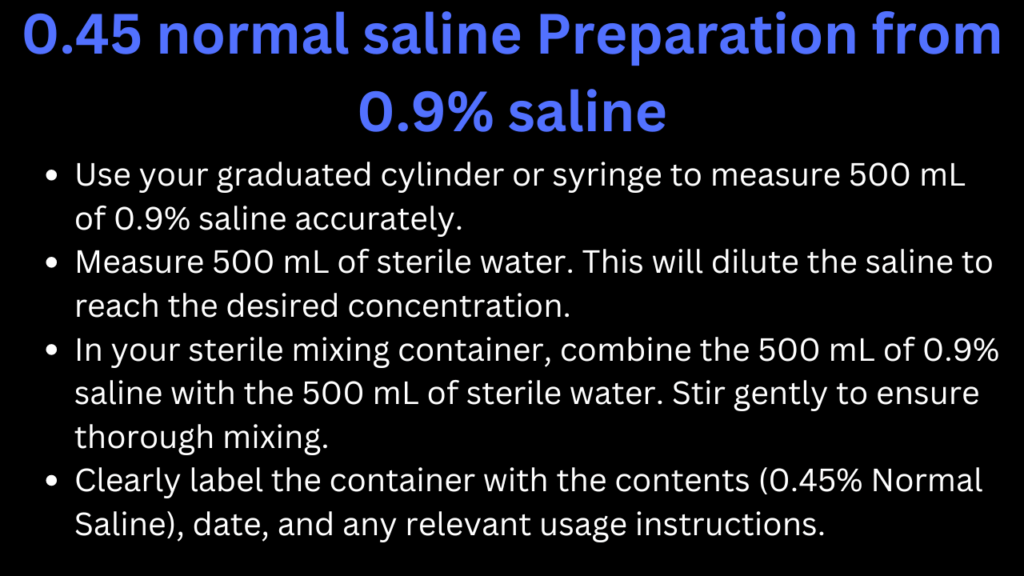
PROCEDURE
- Calculate the Required Volumes:
- To make 1 liter (1000 mL) of 0.45% saline, you can use the dilution formula:
[
C_1V_1 = C_2V_2
]
Where: - (C_1) = concentration of the starting solution (0.9%)
- (V_1) = volume of the starting solution needed
- (C_2) = concentration of the final solution (0.45%)
- (V_2) = volume of the final solution (1000 mL) Rearranging the formula gives:
[
V_1 = \frac{C_2V_2}{C_1} = \frac{0.45\% \times 1000 \, \text{mL}}{0.9\%} = 500 \, \text{mL}
] This means you’ll need 500 mL of 0.9% saline.
- Store the prepared 0.45% saline in a sterile environment. If it’s not going to be used immediately, ensure it’s refrigerated and check for expiration based on your facility’s guidelines.
Important Considerations
- Aseptic Technique: Always maintain a sterile environment to prevent contamination.
- Patient Needs: Ensure that the dilution is appropriate for the patient’s specific medical condition.
- Consult Guidelines: Always follow institutional protocols and guidelines when preparing solutions.