Spectrometers and spectrophotometers are two powerful tools that have revolutionized the field, providing insights into the composition, structure, and properties of substances.
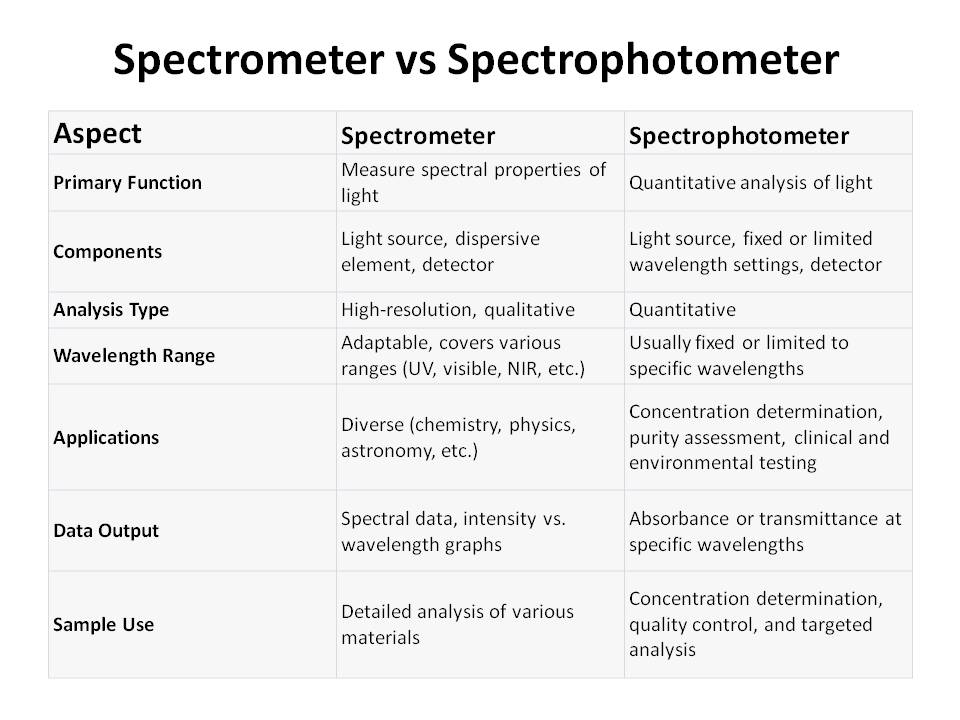
Here is the “difference between spectrometer and spectrophotometer”.
What is Spectrometer
A spectrometer is an instrument designed to measure the spectral properties of light. It works by dispersing light into its constituent colors or wavelengths, which can then be analyzed individually.
The primary components of a spectrometer are a light source, a dispersive element (usually a diffraction grating or prism), and a detector. When light passes through the dispersive element, it spreads out into a spectrum, creating a vivid rainbow of colors.
Spectrometers are commonly used in a wide range of scientific disciplines, including physics, chemistry, and astronomy.
What are the Key Features of Spectrometers
- High-Resolution Analysis: Spectrometers provide detailed information about the different wavelengths of light, allowing for precise analysis of samples. This makes them invaluable in research and quality control.
- Wavelength Flexibility: Spectrometers can be adapted for various wavelength ranges, from ultraviolet (UV) and visible to near-infrared (NIR) and beyond. This adaptability is crucial for analyzing diverse materials.
- Applications: Spectrometers find applications in diverse fields, from identifying chemical compounds to studying celestial objects. They are used in research, industry, and environmental monitoring.
- Spectral Data: Spectrometers yield spectral data, which is often displayed as a graph with intensity on the y-axis and wavelength on the x-axis. Scientists interpret this data to understand the characteristics of the sample.
What is Spectrophotometer
Spectrophotometers are a specific type of spectrometer optimized for quantitative analysis of the absorption or transmission of light by a substance. They are extensively employed in chemistry, biochemistry, and biology for tasks such as quantifying the concentration of a solute in a solution, determining the purity of chemicals, or measuring the absorbance of specific compounds.
What are the Key Features of Spectrophotometers:
- Quantitative Analysis: It is calibrated to provide quantitative measurements, often in terms of absorbance or transmittance. It determine the concentration of a solute in a solution.
- Fixed Wavelengths: On the other hand spectrophotometers are usually designed to operate at specific wavelengths . They are optimized for accuracy in targeted applications.
- Applications: Spectrophotometers are widely used in fields where precise quantitative measurements are crucial, such as clinical laboratories, pharmaceuticals, and environmental testing.
- Spectral Data: Spectrophotometers typically produce simple graphs showing the absorbance or transmittance at a specific wavelength. These measurements are vital for determining the concentration of an analyte.
How does a spectrophotometer work
Here’s a step-by-step explanation of how does spectrophotometer works.
- Light Source: The spectrophotometer begins by emitting a beam of light, typically in the visible or ultraviolet range.
- Monochromator: The emitted light passes through a monochromator, which separates it into different wavelengths by dispersing it, usually with the help of a diffraction grating or prism.
- Sample Analysis: The separated light beam then encounters the sample. The sample may absorb certain wavelengths of light, depending on its composition.
- Detector: A detector, often a photodiode or photomultiplier tube, measures the intensity of the light that passes through the sample.
- Reference Cell: A reference control cell is used to compensate for variations in the light source and other factors.
- Data Comparison: The spectrophotometer compares the intensity of light before and after it passes through the sample.
- Data Display: The spectrophotometer typically displays the results as absorbance values at specific wavelengths, allowing for quantitative analysis of the sample’s composition or concentration.
This process enables the spectrophotometer to provide precise measurements of the absorption or transmission of light, which are crucial for various scientific and analytical applications.
The choice between a spectrometer and a spectrophotometer largely depends on the intended application. If your goal is to obtain detailed spectral information and investigate a wide range of materials, a spectrometer is the way to go. On the other hand, if you require precise quantitative data for concentration measurements or purity assessments, a spectrophotometer is the ideal tool.
In summary, spectrometers and spectrophotometers are both indispensable instruments in the field of analytical chemistry and light analysis. Their differences lie in their intended applications and the types of data they generate. Understanding these distinctions is essential for researchers and analysts to select the right instrument for their specific needs and unlock the secrets of light and matter.
4 thoughts on “Difference between spectrometer and spectrophotometer”